Illuminating new paths to nuclear medicine
At Cyclomedica, our purpose transcends conventional boundaries. We envision a world where, through our innovative technology, we will improve the quality and longevity of the lives of millions of respiratory patients through early intervention and improved patient management care.
Three guiding principles fuelling our passion
Aspire
We ‘Aspire’ to elevate the industry standards, relentlessly innovating and striving to deliver the finest products and services in nuclear pulmonology.
Inspire
We aim to ‘Inspire’ everyone we work with – our customers, partners, and patients – providing them the tools to overcome professional and personal challenges.
Illuminate
We ‘Illuminate’, guiding the way towards a healthier future by delivering transformative, innovative solutions in respiratory disease management.
Our vision for success: Amplifying health outcomes and setting new pulmonary care benchmarks with Technegas®

We empower healthcare providers and patients across the globe with Technegas®, our state-of-the-art diagnostic lung imaging platform. As we look to the future, our four-pillar strategy will continue to guide our journey:
- GROWING:
The use of Technegas in existing Pulmonary Embolism markets - EXPANDING:
The application of Technegas beyond Pulmonary Embolism - ACCELERATING:
Partners’ product distribution strategies; and finally - INNOVATING:
Complementary technologies
Through our persistent dedication to innovation and our extended network, our goal is to make Technegas® accessible to every patient who needs it, ensuring timely diagnoses and vital health assessments for the best possible outcomes. This ambitious aspiration reflects our determination to drive growth, elevate performance, and set new benchmarks in pulmonary care.
Downloadable content
Technegas brochure

Our core values
Guided by our principles, Cyclomedica is committed to revolutionising pulmonary care. These core values inspire our work, shape our culture, and define who we are:
Quality, integrity, and efficacy
By upholding the highest standards of ethics, excellence, integrity and quality; we ensure that our solutions are not just effective but also consistently reliable, earning the trust of partners and patients.
Humanity and patient-centricity
The patient is at the core of every aspect of our work. By placing the patient at the centre of our mission we ensure ensuring their health, safety, and well-being are prioritised.
Innovation and safety
We harness the power of innovation to drive progress while upholding the sanctity of patient safety. Our commitment to continuous improvement ensures we remain at the forefront of pulmonary care.
Collaboration and respect
Fostering collaborative partnerships is integral to us. We respect and empower all our stakeholders, recognising that our shared success is pivotal to advancing global healthcare.
Accountability and responsibility
Acknowledging the significance of our role in the industry, we approach every decision with a profound sense of responsibility, ensuring we hold ourselves accountable for the impact of our actions.
Fostering a culture of collaboration for collective success
Cyclomedica’s success is gauged by our collective impact on the communities we serve. From healthcare professionals who rely on our solutions, to the patients whose lives we strive to improve, to the partners alongside us on this transformative path, everyone plays a crucial part in our story. Our mission is to foster an environment where exceptional talent can flourish, innovate, and make a significant impact on the patients we serve globally.
We place immense value on cultivating strong, enduring relationships with our trusted partners and customers, understanding that their successes are fundamental to our own.
Historical milestones
Explore the pivotal moments in Cyclomedica’s history, a testament to decades marked by innovation, growth, and impactful achievements.











1984
Experimentation at Canberra University on a novel method of combining carbon nanoparticles with radioactive isotopes
1986
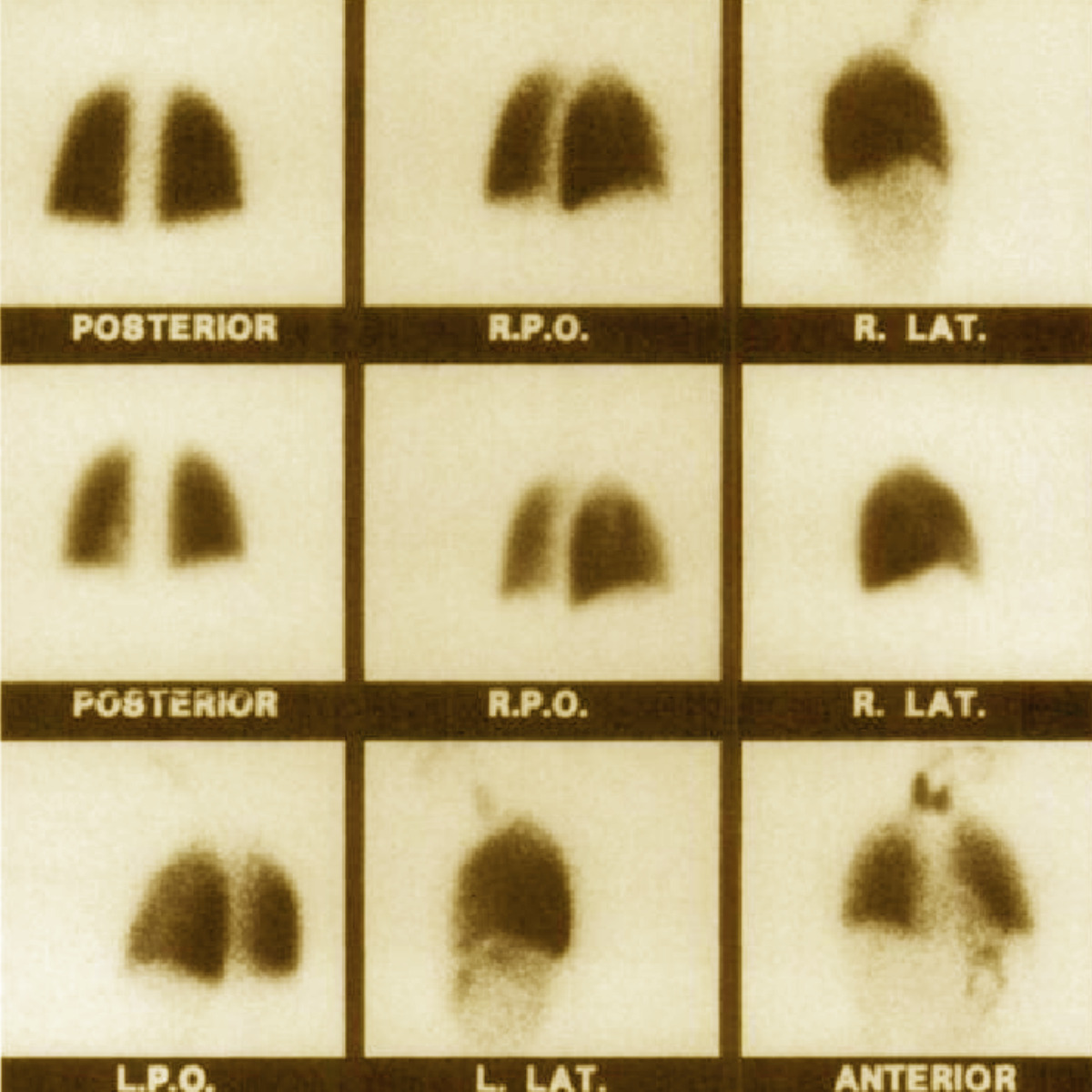
Technegas first approved for clinical use in Australia
1987
Technegas launched in the European market starting with the UK
1994
Technegas launched in Asia
1995
Pertechnegas – a Technegas variation was developed
2000
Galligas – a P.E.T. variation of Technegas using Ga-68 is developed
2001
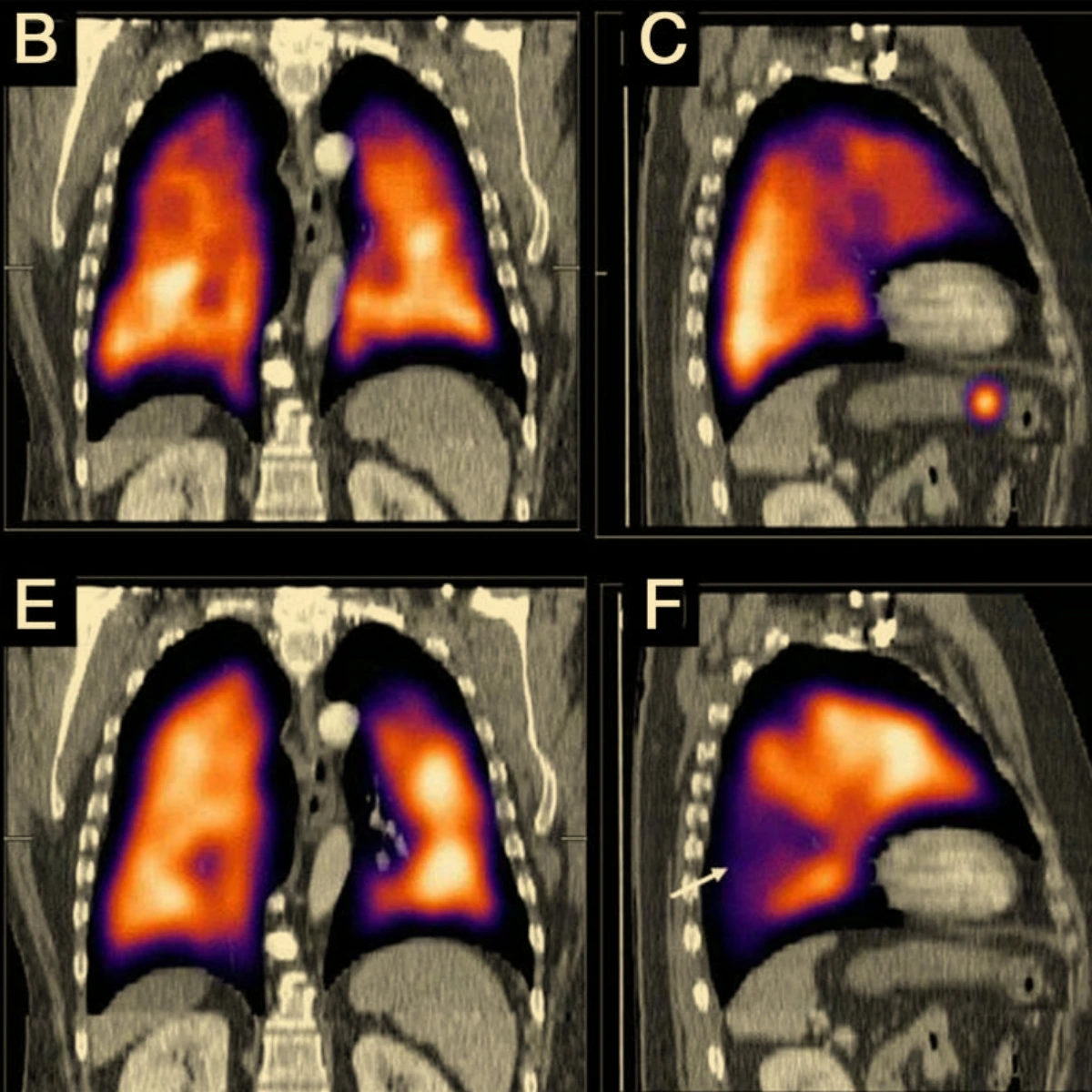
Three dimensional SPECT Imaging introduced in nuclear medicine and opens doors for broader applications in lung imaging
2005
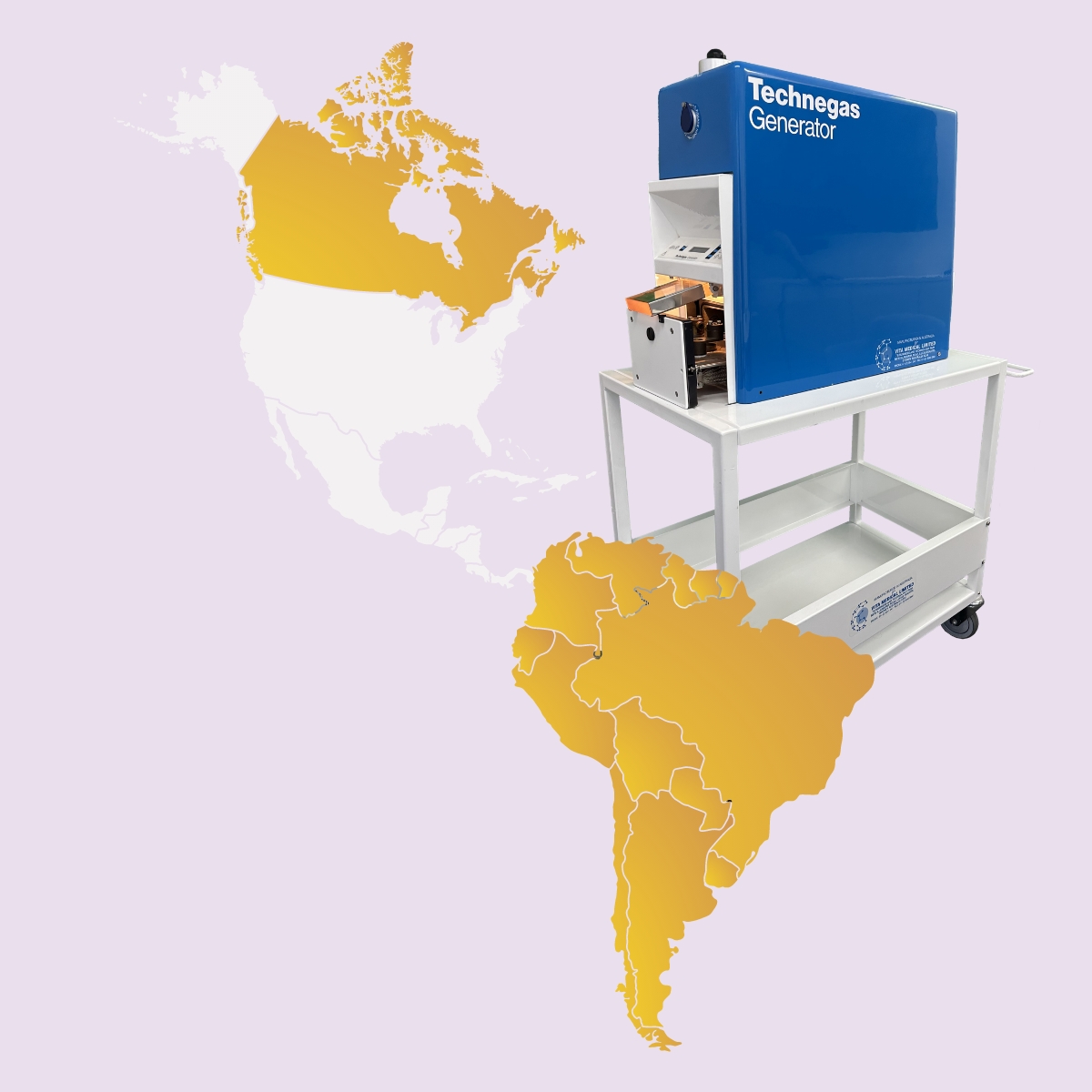
Technegas launched in Canada and South America
2006
TechnegasPlus, the new generation of Technegas Generator is launched globally
2009
Technegas named in the European Association of Nuclear Medicine Guidelines as the preferred functional lung ventilation imaging agent
2018
Canada becomes the largest market for Technegas
2019
Technegas named in the Canadian Association of Nuclear Medicine Guidelines as the preferred functional lung ventilation imaging agent
2020
USFDA Clinical trial completed and New Drug Application Submitted to USFDA
2023
Technegas gains approval for distribution in the USA